Publication in Inorganic Chemistry
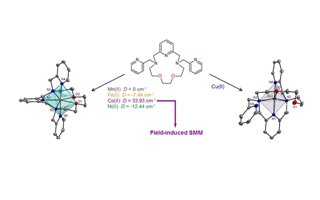 The 2-pyridylmethyl N-pendant armed heptadentate macrocyclic ligand {3,12-bis(2-methylpyridine)-3,12,18-triaza-6,9-dioxabicyclo[12.3.1]octadeca-1,14,16-triene = L} and [M(L)](ClO4)2 complexes, where M = Mn(II) (1), Fe(II) (2), Co(II) (3), Ni(II) (4) and Cu(II) (5), were prepared and thoroughly characterized, including elucidation of X-ray structures of all the compounds studied. The complexes 1–5 crystallize in non-centrosymmetric Sohncke space groups as racemic compounds. The coordination number of 7, 6+1 and 5 was found in complexes 1–3, 4, and 5, respectively, with a distorted pentagonal bipyramidal (1–4) or square pyramidal (5) geometry. Based on the magnetic susceptibility experiments, a large axial zero-field splitting (ZFS) was found for 2, 3 and 4 (D(Fe) = –7.4(2) cm–1, D(Co) = 34(1) cm–1, and D(Ni) = –12.8(1) cm–1, respectively) together with a rhombic ZFS (E/D = 0.136(3)) for 4. Field-induced single-molecule magnet (SMM) behavior with easy plane anisotropy was observed for 3, and the relaxation time τ0 = 9.90 × 10−10 s and spin reversal barrier Ueff = 24.3 K (16.9 cm–1) were obtained together with the indication of Orbach and Raman relaxation processes. The cyclic voltammetry in acetonitrile revealed reversible redox processes in 1–3 and 5, except for the Ni(II) complex 4 where a quasi-reversible process was dominantly observed. Presence of the two 2-pyridylmethyl pendant arms in L with a stronger s-donor/π-acceptor ability had a great impact on the properties of all the complexes (1–5), concretely: (i) strong pyridine–metal bonds provided slight axial compression of the coordination sphere, (ii) substantial changes in magnetic anisotropy, and (iii) stabilization of lower oxidation states.
The 2-pyridylmethyl N-pendant armed heptadentate macrocyclic ligand {3,12-bis(2-methylpyridine)-3,12,18-triaza-6,9-dioxabicyclo[12.3.1]octadeca-1,14,16-triene = L} and [M(L)](ClO4)2 complexes, where M = Mn(II) (1), Fe(II) (2), Co(II) (3), Ni(II) (4) and Cu(II) (5), were prepared and thoroughly characterized, including elucidation of X-ray structures of all the compounds studied. The complexes 1–5 crystallize in non-centrosymmetric Sohncke space groups as racemic compounds. The coordination number of 7, 6+1 and 5 was found in complexes 1–3, 4, and 5, respectively, with a distorted pentagonal bipyramidal (1–4) or square pyramidal (5) geometry. Based on the magnetic susceptibility experiments, a large axial zero-field splitting (ZFS) was found for 2, 3 and 4 (D(Fe) = –7.4(2) cm–1, D(Co) = 34(1) cm–1, and D(Ni) = –12.8(1) cm–1, respectively) together with a rhombic ZFS (E/D = 0.136(3)) for 4. Field-induced single-molecule magnet (SMM) behavior with easy plane anisotropy was observed for 3, and the relaxation time τ0 = 9.90 × 10−10 s and spin reversal barrier Ueff = 24.3 K (16.9 cm–1) were obtained together with the indication of Orbach and Raman relaxation processes. The cyclic voltammetry in acetonitrile revealed reversible redox processes in 1–3 and 5, except for the Ni(II) complex 4 where a quasi-reversible process was dominantly observed. Presence of the two 2-pyridylmethyl pendant arms in L with a stronger s-donor/π-acceptor ability had a great impact on the properties of all the complexes (1–5), concretely: (i) strong pyridine–metal bonds provided slight axial compression of the coordination sphere, (ii) substantial changes in magnetic anisotropy, and (iii) stabilization of lower oxidation states.


