A new strategy will allow the synthesis of novel polymers
Czech scientists, in collaboration with colleagues from Spain, introduced in the journal Nature Communications a new kind of polymers that were previously unthinkable. This kind of polymer can play a significant role in the design of new components for nanoelectronics, such as new displays. At the same time, the researchers demonstrated that molecular vibrations significantly influence the course of reactions on the surfaces of solids and can be used for targeted synthesis of conductive polymers.
Scientists from the Regional Centre of Advanced Technologies and Materials (RCPTM) of Palacký University Olomouc and the Institute of Physics of the CAS together with Spanish colleagues from the research centre IMDEA Nanociencia, Madrid, developed a new synthetic strategy which has surpassed traditional organic synthesis practices.
“This procedure enables us to prepare new polymers in a novel way, which will also shine line on well-established ideas about reaction mechanisms,” commented on the achievement Pavel Jelínek from RCPTM and the Institute of Physics of the CAS.
The prospective route to preparing new organic molecular-based nanomaterials is a chemical synthesis on the surfaces of solids, allowing the preparation of conductive organic polymer compounds unavailable via classical organic synthesis procedures. Most chemical reactions to create a new chemical compound require overcoming a certain energy barrier. The new strategy also enables the use of suitable internal vibrations of molecular units to overcome this barrier.
“We demonstrated in the article that specific internal bonding arrangements, called π-conjugations, of molecules entering the reaction correspond to certain vibrational states that can be beneficial for the desired chemical reaction. These in turn significantly increase the frequency of attempts to overcome the energy barrier and allow a chemical reaction that would have been previously ruled out,” said the corresponding author of the study Bruno de la Torre from RCPTM and the Institute of Physics of the AV CR.
The work of the international team thus reveals the importance of internal molecular vibrations for understanding chemical reactions on the surfaces of solids. The feasibility of a chemical reaction is due to two factors—the barrier to the activation energy of the reaction and the frequency of vibration modes that allow the energy barrier to be overcome.
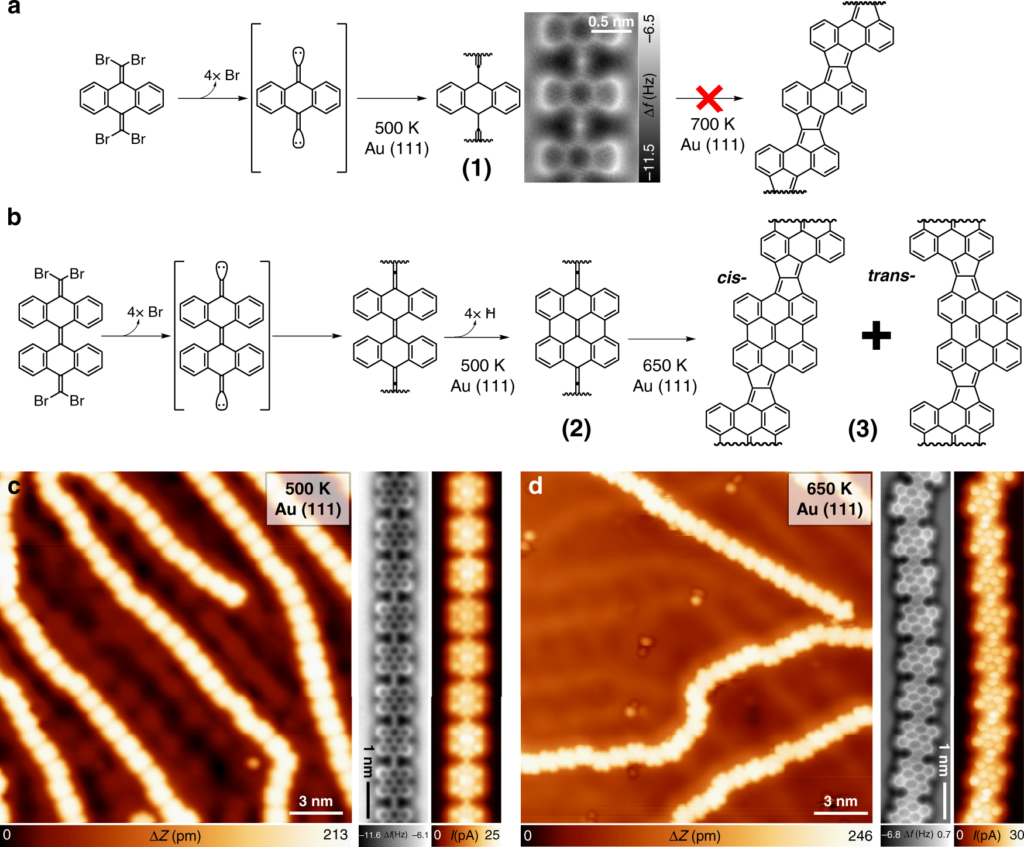
At the top: schematic of a two-stage chemical reaction that allows the synthesis of a ladder polymer (model on the right). At the bottom: image from a high-resolution raster microscope showing the chemical structure of the intermediate (left) and final (right) ladder polymer
New alternative—accelerating some chemical reactions
The current description of the rate of chemical reactions is based on the so-called transition state theory, which, in its basic form, defines the rate of reaction only on the basis of the size of the activation energy. So most chemists have focused on studying activation energy barriers in an effort to reduce them so that a chemical reaction can take place successfully. Common practice is to increase the temperature or add a catalyst. Another alternative may be the possibility of accelerating some chemical reactions by increasing the frequency of attempts to overcome the energy barrier.
“Combining microscopic experiments and theoretical calculations, we have confirmed that by choosing a specific form of the reactant’s π-conjugation, i.e., a molecule entering the reaction, vibration modes can be achieved, which will significantly increase the frequency of attempts and allow a previously impossible chemical reaction,” explained the PhD student Adam Matěj from RCPTM and the Institute of Physics.
Scientists have accurately defined the electron structure of the polymer
Another benefit of the work is that the researchers, using high-resolution atomic force microscopy, were able to accurately determine the electron structure of the polymer. “Clarification of the electron structure of organic conductive nanomaterials is essential to understanding their conductivity properties, for example. New methods of surface synthesis then open the door to the targeted synthesis of organic polymers with the required conductivity,” added Michal Otyepka from RCPTM.
Therefore, a new synthetic strategy based on the use of specific vibration modes via the appropriate adaptation of the π-conjugation of precursors may spur engineering of previously impracticable reactions of new molecular structures with specific chemical and physical properties.
Article link: https://www.nature.com/articles/s41467-020-18371-2


