Publication in Inorganica Chimica Acta
Lanthanide complexes containing pentadentate s-triazine-based Schiff base ligand: Synthesis, structure and magnetic features
Authors: Ján Vančo, Zdeněk Trávníček
Full-text: https://doi.org/10.1016/j.ica.2025.122690
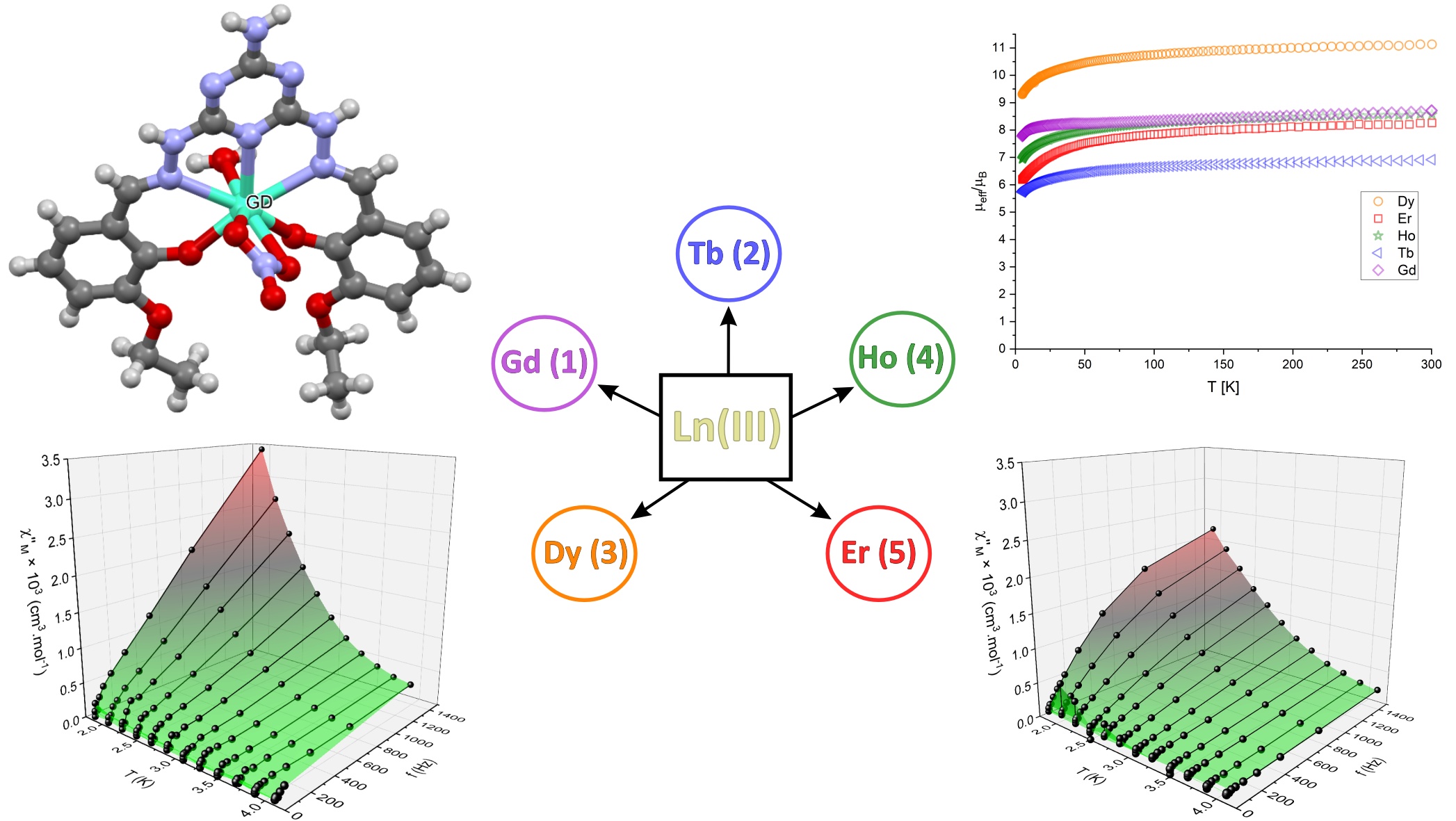
Five mononuclear Ln(III) complexes {Ln = Gd (1), Tb (2), Dy (3), Ho (4) and Er (5)} of the general composition [Ln(L)(NO3)(H2O)]⋅solv (solv = H2O and/or DMF) were synthesized and thoroughly characterized. The pentadentate H2L Schiff base ligand, formed by condensation of 2,4-bishydrazino-6-amino-s-triazine with 3-ethoxysa- licylaldehyde, is coordinated to the Ln(III) atoms in the N3O2 donor set in an equatorial plane. The coordination geometry of the central metal atoms is then completed by a bidentate-bonded nitrato ligand and one water molecule, thus forming a biaugmented trigonal prism environment, as follows from the X-ray structure of complex 1. A detailed magnetic evaluation (static (dc) and dynamic (ac) data) of complexes 1–5 uncovered that they behave as magnetically diluted paramagnetics with weak antiferromagnetic features originating probably in the presence of non-covalent bonding in the solid samples. Only the complexes of Dy (3) and Er (5) showed signs of slow relaxation of magnetization with the estimated energy barrier values of Ueff = 9.4 (5) K (for 3) and Ueff = 9.9 (6) K (for 5), and relaxation times of τ0 = 0.25 (8) μs (for 3) and τ0 = 0.45(9) μs (for 5).