Publication in Journal of Inorganic Biochemistry
Copper(II) tetrapyrazole-based complex as a new peroxidase-mimetic compound
Authors: Marek Šebela, Giorgio Zoppellaro, Zdeněk Trávníček
Full-text: https://doi.org/10.1016/j.jinorgbio.2025.112911
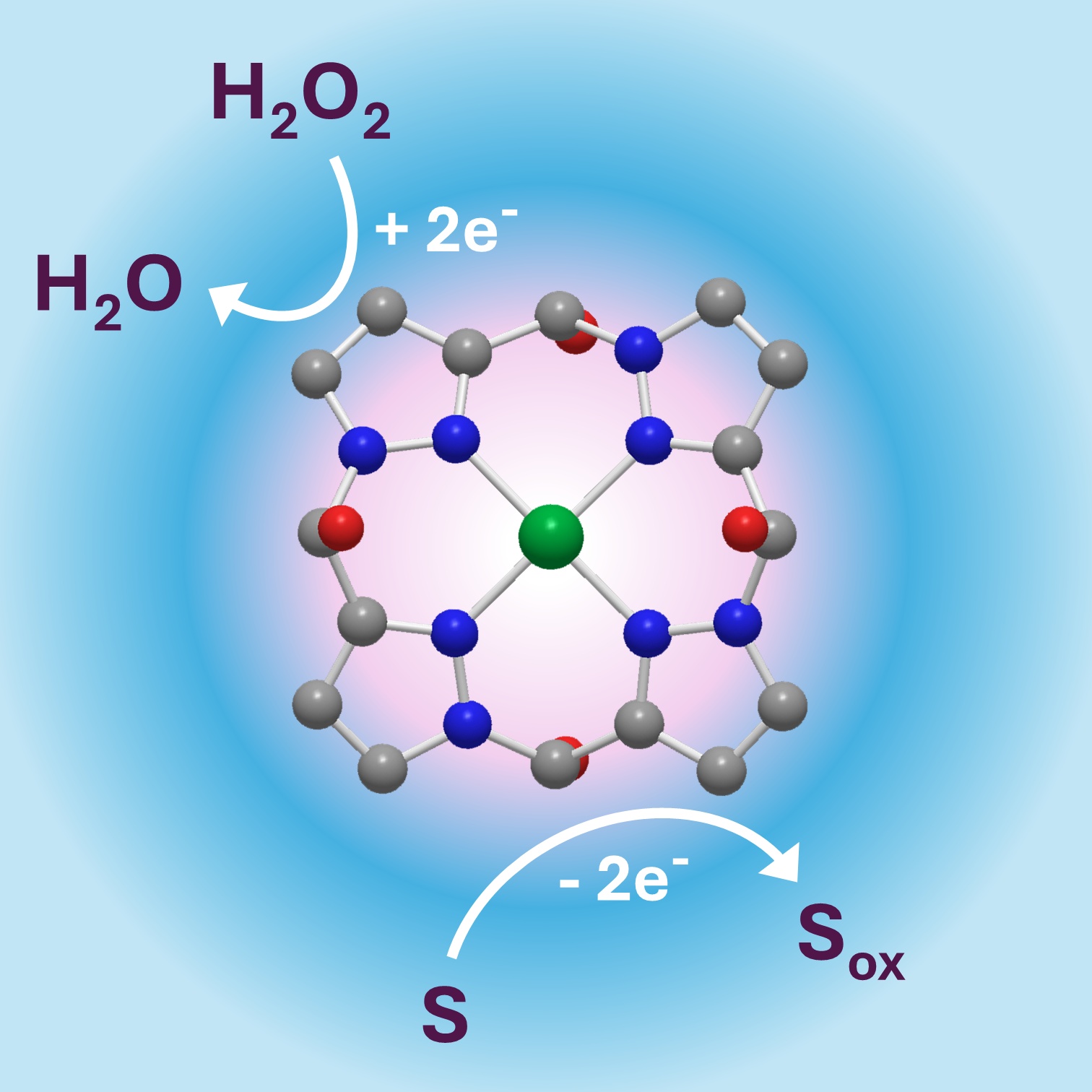
A copper(II) tetrapyrazole-based complex of the composition of [Cu(tpyr)(H2O)(ONO2)]NO3 (1), where tpyr represents a tetradentate N-donor ligand formed by the condensation of 1H-pyrazole-5-carbaldehyde in NaOH/ MeOH medium, has been prepared and characterized by elemental analysis, infrared spectroscopy, ultraviolet- visible spectroscopy, mass spectrometry, electron paramagnetic resonance and single-crystal X-ray diffraction. Spectrophotometric measurements demonstrated a remarkable peroxidase activity of the complex, which utilized hydrogen peroxide for the oxidation of phenolic compounds such as guaiacol or 3,5-dichloro-2-hydroxybenzene sulfonic acid. The optimum conditions for this reaction were found at pH 8 in ammonium bicarbonate buffer, although the activity was low but still detectable at pH 5–6 in ammonium acetate. As a peroxidase mimic, the complex exhibited enzyme-like Michaelis-Menten kinetics, showing a hyperbolic dependence of the reaction rate on hydrogen peroxide concentration. The determined Km and kcat values were 651 μmol⋅l-1 and 6.7 × 10-4 s-1, respectively, compared to 41 μmol⋅l-1 and 73 s-1 for horseradish peroxidase. EPR spectroscopy of the reaction mixture revealed no change in the copper (II) oxidation state during catalysis, suggesting that the oxidation of guaiacol may occur simultaneously with the reduction of hydrogen peroxide to water at the copper centre.