Scientists have provided new insights into interaction of proteins with RNA
An international scientific consortium involving researchers from RCPTM and the Institute of Biophysics of the Czech Academy of Sciences has yielded the insight into HuR (Human antigen R) protein, elevated levels of which were spotted in various diseases. Not only do the research results provide general information on the interaction of these specific proteins with RNA, but they could also contribute to designing novel medical treatments. A paper called Molecular basis for AU-rich element recognition and dimerization by the HuR C-terminal RRM was recently published in the journal Proceedings of the National Academy of Sciences (PNAS).
The interaction of proteins with RNA is an essential molecular process, which has shaped the evolution since the first RNA living molecules up to today’s organisms. In all existing biological systems, except simplest viroids, all functional RNA molecules always interact with proteins in the course of their entire existence. In fact, in case a living cell identifies a free RNA molecule inside, it immediately destroys it. This is a natural defence against ubiquitous RNA viruses. A wide range of proteins specifically designed to interact with RNA has evolved so that cells will not damage their own RNA molecules, and will be able to regulate them. An example of such a protein is RRM (RNA recognition motif) domain. In general, the binding of the RRM domain to RNA is very specific, thus allowing the protein to identify the particular RNA sequence. These domains are responsible for a good half of all protein-RNA interactions in more complex organisms.
“In our work, we focused on researching HuR protein, which plays a key role in the regulation of mRNA sequences that are rich in adenine and uracil, so called AU sequence. HuR contains three RRM domains; however, the structure and function of the third one was not solved until recently. By means of X-ray crystallography, our colleagues from ETH Zurich and Novartis, a pharmaceutical company, succeeded in creating an atomic model of the third RRM domain of HuR binding RNA,” said Jiří Šponer, from the Institute of Biophysics of the Czech Academy of Sciences and RCPTM. According to him, the structure revealed continuous movement of the bound RNA, which hindered accurate localization of particular nucleotides. “In our laboratory, employing molecular dynamics, we were able to demonstrate that this RRM domain is capable of identifying RNA by means of mobile and constantly changing interactions with protein. Understanding of such dynamic system is very difficult to achieve by experimental methods as they usually provide a static image of the particular molecule, excluding any information about its movement. The study on the binding to RNA further showed that this RRM domain is able to bind both AU sequences and sequences containing solely uracil. One of the outcomes of dynamic recognition is the fact that selected binding sites on the surface of the domain are capable of binding uracil and adenine, while others bind solely uracil,” said Miroslav Krepl, one of the authors of the study.
These findings significantly contribute to understanding of the HuR protein, elevated levels of which were spotted in various diseases. The insight into the unique way of RNA recognition employed by the third RRM domain and its sequence specificity therefore offers the possibility of designing novel medical treatments. “Given the biological significance of RRM domains, our study also introduces a general mechanism by which these versatile molecules can recognize RNA. Dynamic recognition represents a unique evolutionary response to situations in which specific interactions with RNA are vital, but at the same time the cell needs to rapidly switch between a number of various RNA sequences. Such a unique way of interaction differs a great deal from the current standard model in which rigid non-covalent bonding occurs between the protein and RNA, concluded Šponer.
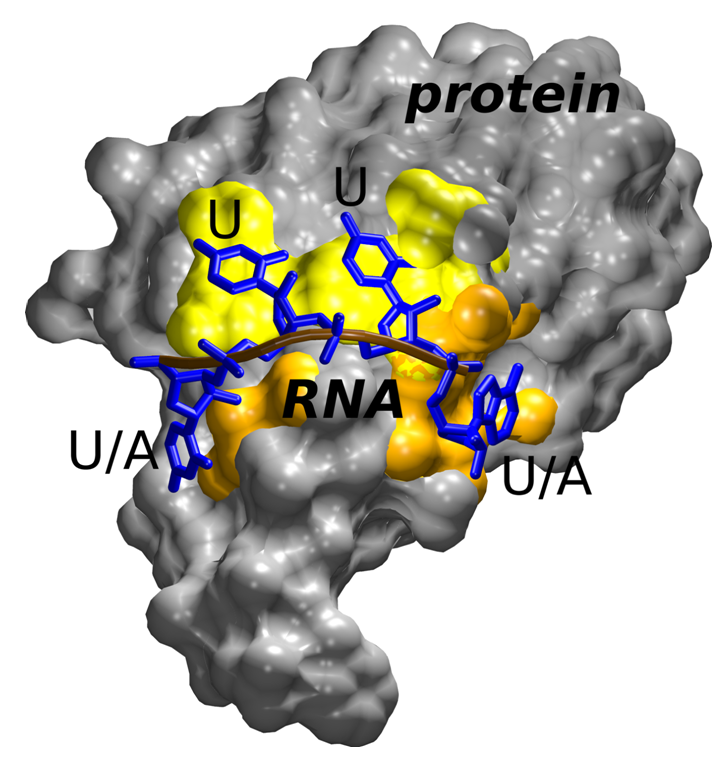
Figure 1 The third RRM domain of HuR protein (grey) involving classical RNA (blue) binding sites recognizing uracil only (yellow); further, unique binding sites capable of accepting either uracil or adenine (orange).


