Researchers Enhanced Ammonia Production Process
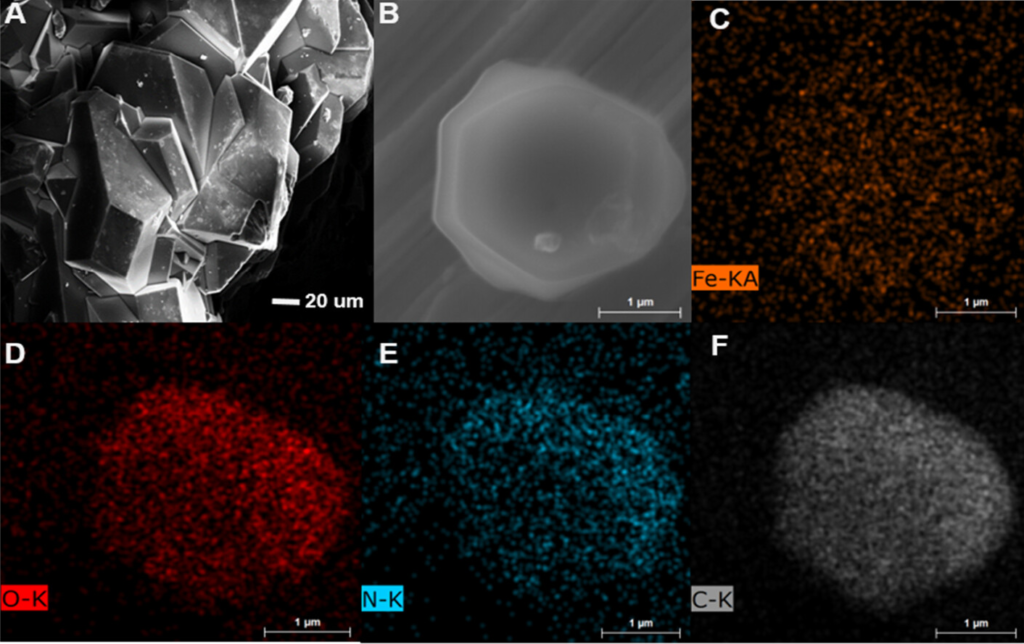
A significant step towards achieving more efficient and sustainable ammonia production was unveiled by scientists from CEITEC Energy at Brno University of Technology, CATRIN of Palacký University Olomouc, and the IT4Innovations National Supercomputer Center at VSB-TUO. Their findings, published in the esteemed journal ACS Applied Materials & Interfaces, showcase the electrochemical reduction of nitrate to ammonia. The catalyst employed in this process is an activated material based on metal-organic frameworks (MOF). These materials have demonstrated exceptional efficacy in optimizing the energy intensity of several industrially vital reactions. In this specific case, the scientists proved this through a combination of theoretical calculations and experimental validation.
Electrochemical reduction of nitrate to ammonia is a promising solution to meeting the challenges posed by increasing energy demand and efforts to reduce negative environmental impacts. Nitrates, which are a common pollutant in waters for example, can also serve as a suitable source of nitrogen in ammonia synthesis. Ammonia is a major industrial product, but its production is very energy intensive, with an impact of around 2% on global carbon dioxide emissions. Scientists have therefore focused their attention on exploring new electrocatalytic materials that can accelerate the conversion of nitrate to ammonia.
“Metal-organic frameworks (MOFs) are intensively studied materials that enable a wide range of applications. Our team focused on the ability of iron-containing MOFs to catalyse the electrochemical conversion of nitrates to ammonia. Colleagues from Brno showed that the studied catalyst achieves a staggering 90% efficiency and thus pushes the boundaries in the field of electrocatalysis. The mechanism was elucidated by sophisticated computer simulations on KAROLINA—currently the most powerful supercomputer in the Czech Republic. Understanding the mechanism offers a unique insight that can be used to further improve this class of catalysts in the future,” said one of the authors of the article Michal Otyepka from CATRIN and IT4Innovations.
The study clearly shows how the findings from experiments and computer simulations complement each other excellently. The integration of computational and experimental approaches is one of the pillars of the TECHSCALE project, which aims to develop new materials and approaches that will significantly extend the current frontiers of nanomaterials research to the design of new materials with atomic precision.
“Thanks to the great collaboration, we have gained comprehensive information not only on the behaviour, but especially on the further potential of this material. These are important findings for a more efficient and sustainable reduction of waste nitrates to ammonia, but they also open up new possibilities for researchers who would like to modify or surface-modify materials to prepare electrocatalysts for other industrially important catalytic reactions,” emphasised Martin Pumera from CEITEC Energy in Brno, who led the research.
The publication is also one of the first results of the TECHSCALE project, which received almost 500 million CZK of funding in the Excellent Research Call from the Jan Amos Komenský Operational Programme and achieved the second highest ranking in the competition of almost a hundred projects. The aim of the researchers is to develop new nanomaterials and technologies that will contribute to solving two current global challenges—harnessing and storing renewable energy and developing new materials for the improvement of quality of life. The project will also include social impact assessment and public acceptance of new technologies. The project will be implemented under the leadership of the physical chemist Michal Otyepka and will bring together researchers from CATRIN and five faculties of Palacký University with scientists from Charles University and CEITEC VUT.